Linking crystal structure with temperature-sensitive vibrational modes in calcium carbonate minerals
Abstract
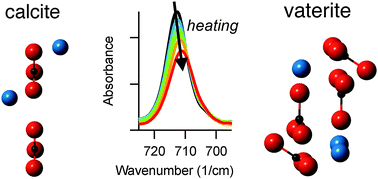
We demonstrate a correlation between how an IR-active vibrational mode responds to temperature changes and how it responds to crystallinity differences. Infrared (IR) spectroscopy was used to track changes in carbonate-related vibrational modes in three different CaCO3 polymorphs (calcite, aragonite, and vaterite) and CaMg(CO3)2 (dolomite) during heating. Of the three characteristic IR-active carbonate modes, the in-plane bending mode (ν4) shows the most pronounced changes with heating in polymorphs that have planar carbonate arrangements (calcite, aragonite, and dolomite). In contrast, this mode is virtually unchanged in vaterite, which has a canted arrangement of carbonate units. We correlate these trends with recent studies that identified the ν4 mode as most susceptible to changes related to crystallinity differences in calcite and amorphous calcium carbonate. Thus, our results suggest that studies of packing arrangements could provide a generalizable approach to identify the most diagnostic vibrational modes for tracking either temperature-dependent or crystallinity-related effects in IR-active solids.

 Please wait while we load your content...
Please wait while we load your content...