A quantum chemical study on gas phase decomposition pathways of triethylgallane (TEG, Ga(C2H5)3) and tert-butylphosphine (TBP, PH2(t-C4H9)) under MOVPE conditions†
Abstract
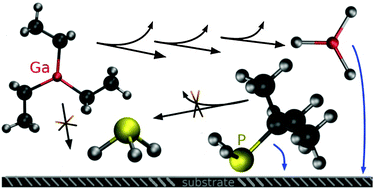
The gas phase decomposition reactions of precursor molecules relevant for metal–organic vapour phase epitaxy (MOVPE) of semiconductor thin films are investigated by computational methods on the density-functional level as well as on the ab initio (MP2, CCSD(T)) level. A comprehensive reaction catalogue of uni- and bimolecular reactions is presented for triethylgallium (TEG) as well as for tert-butylphosphine (TBP) containing thermodynamic data together with transition state energies. From these energies it can be concluded that TEG is decomposed in the gas phase under MOVPE conditions (T = 400–675 °C, p = 0.05 atm) to GaH3via a series of β-hydride elimination reactions. For elevated temperatures, further decomposition to GaH is thermodynamically accessible. In the case of TBP, the original precursor molecule will be most abundant since all reaction channels exhibit either large barriers or unfavorable thermodynamics. Dispersion-corrected density functional calculations (PBE-D3) provide an accurate description of the reactions investigated in comparison to high level CCSD(T) calculations serving as benchmark values.

 Please wait while we load your content...
Please wait while we load your content...