Gas-phase electrophilic aromatic substitution mechanism with strong electrophiles explained by ab initio non-adiabatic dynamics†
Abstract
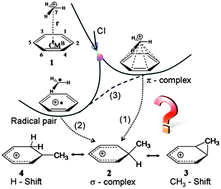
Ab initio non-adiabatic dynamics is used to monitor the attack of CH3+ to benzene. The results show that in the gas phase the reaction is ultrafast and is governed by a single electron transfer producing a radical pair.

 Please wait while we load your content...
Please wait while we load your content...