Carbon dioxide interaction with isolated imidazole or attached on gold clusters and surface: competition between σ H-bond and π stacking interaction†
Abstract
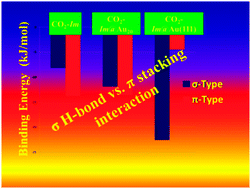
Using first principle methodologies, we investigate the subtle competition between σ H-bond and π stacking interaction between CO2 and imidazole either isolated, adsorbed on a gold cluster or adsorbed on a gold surface. These computations are performed using MP2 as well as dispersion corrected density functional theory (DFT) techniques. Our results show that the CO2 interaction goes from π-type stacking into σ-type when CO2 interacts with isolated imidazole and Au clusters or surface. The balance between both types of interactions is found when an imidazole is attached to a Au20 gold cluster. Thus, the present study has great significance in understanding and controlling the structures of weakly-bound molecular systems and materials, where hydrogen bonding and van der Waals interactions are competing. The applications are in the fields of the control of CO2 capture and scattering, catalysis and bio- and nanotechnologies.

 Please wait while we load your content...
Please wait while we load your content...