Competition between excited state proton and OH− transport via a short water wire: solvent effects open the gate†
Abstract
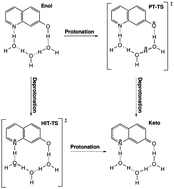
We investigate the acid–base proton exchange reaction in a microsolvated bifunctional chromophore by means of quantum chemical calculations. The UV/vis spectroscopy shows that equilibrium of the keto- and enol-forms in the electronic ground state is shifted to the keto conformation in the excited state. A previously unknown mechanism involving a hydroxide ion transport along a short water wire is characterized energetically, which turns out to be competitive with the commonly assumed proton transport. Both mechanisms are shown to have a concerted character, as opposed to a step-wise mechanism. The alternative mechanism of a hydrogen atom transport is critically examined, and evidence for strong solvent dependence is presented. Specifically, we observe electrostatic destabilization of the corresponding πσ* state by the aqueous solvent. As a consequence, no conical intersections are found along the reaction pathway.

 Please wait while we load your content...
Please wait while we load your content...