Characterisation of the electronic structure of some stable nitroxyl radicals using variable energy photoelectron spectroscopy
Abstract
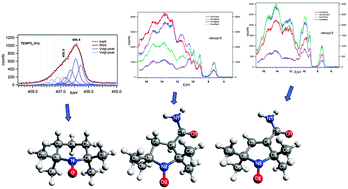
The photoionization of three stable nitroxyl radicals has been studied in the valence and core regions using synchrotron radiation. We observed different variations of the relative band intensities with the photon energy for two pyrrolidine nitroxyls (nitroxyl8 and nitroxyl9) in the valence ionization region. This is due to strong intramolecular interactions between the amide substituent and the ring π-orbital when present. In the core ionization region we observed chemical shifts which were consistent with the relative electron affinities of different atoms. We also observed the multiplet splitting of core level binding energies in the final ionic states. The core electron binding energies calculated via the restricted open shell Hartree–Fock based ΔSCF method exhibit good agreement with the experimental core ionization bands and with the assignment of the spectra by empirical analysis.

 Please wait while we load your content...
Please wait while we load your content...