Hydrogen reversibility of LiBH4–MgH2–Al composites†
Abstract
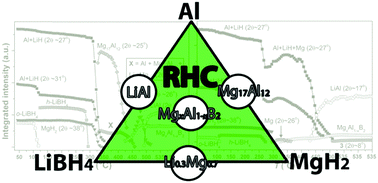
The detailed mechanism of hydrogen release in LiBH4–MgH2–Al composites of molar ratios 4 : 1 : 1 and 4 : 1 : 5 are investigated during multiple cycles of hydrogen release and uptake. This study combines information from several methods, i.e., in situ synchrotron radiation powder X-ray diffraction, 11B magic-angle spinning (MAS) NMR, Sievert's measurements, Fourier transform infrared spectroscopy and simultaneous thermogravimetric analysis, differential scanning calorimetry and mass spectroscopy. The composites of LiBH4–MgH2–Al are compared with the behavior of the LiBH4–Al and LiBH4–MgH2 systems. The decomposition pathway of the LiBH4–MgH2–Al system is different for the two investigated molar ratios, although it ultimately results in the formation of LiAl, MgxAl1−xB2 and Li2B12H12 in both cases. For the 4 : 1 : 1-molar ratio, Mg0.9Al0.1 and Mg17Al12 are observed as intermediates. However, only Mg is observed as an intermediate in the 4 : 1 : 5-sample, which may be due to an earlier formation of MgxAl1−xB2, reflecting the complex chemistry of Al–Mg phases. Hydrogen release and uptake reveals a decrease in the hydrogen storage capacity upon cycling. This loss reflects the formation of Li2B12H12 as observed by 11B NMR and infrared spectroscopy for the cycled samples. Furthermore, it is shown that the Li2B12H12 formation can be limited significantly by applying moderate hydrogen partial pressure during decomposition.

 Please wait while we load your content...
Please wait while we load your content...