Understanding the chemical dynamics of the reactions of dicarbon with 1-butyne, 2-butyne, and 1,2-butadiene – toward the formation of resonantly stabilized free radicals†
Abstract
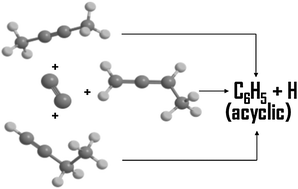
The reaction dynamics of the dicarbon radical C2(a3Πu/X1Σg+) in the singlet and triplet state with C4H6 isomers 2-butyne, 1-butyne and 1,2-butadiene were investigated at collision energies of about 26 kJ mol−1 using the crossed molecular beam technique and supported by ab initio and RRKM calculations. The reactions are all indirect, forming C6H6 complexes through barrierless additions by dicarbon on the triplet and singlet surfaces. Isomerization of the C6H6 reaction intermediate leads to product formation by hydrogen loss in a dicarbon–hydrogen atom exchange mechanism forming acyclic C6H5 reaction products through loose exit transition states in overall exoergic reactions.

 Please wait while we load your content...
Please wait while we load your content...