Physicochemical properties of N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide for sodium metal battery applications
Abstract
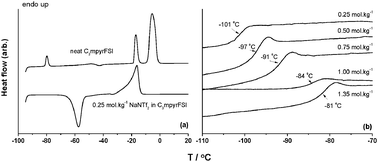
The physicochemical properties of a range of NaNTf2 (or NaTFSI) salt concentrations in N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide (or C3mpyrFSI) ionic liquid were investigated by DSC, conductivity, cyclic voltammetry and diffusivity studies. Cyclic voltammetry indicated a stable sodium plating behavior with a current of 5 mA cm−2 at 25 °C to 20 mA cm−2 at 100 °C, along with high reversibility identifying this electrolyte as a possible candidate for sodium-ion or sodium metal battery applications. 23Na NMR chemical shifts and spectral linewidths (FWHM) indicate a complex coordination of the Na+ ion which is dependent on both temperature and salt concentration with an apparently stronger coordination to the NTf2 anion upon increasing the NaNTf2 concentration. Temperature dependent PFG-NMR diffusion measurements show that both FSI and NTf2 have a comparable behaviour although the smaller FSI anion is more diffusive.

 Please wait while we load your content...
Please wait while we load your content...