Non-symmetric 9,10-diphenylanthracene-based deep-blue emitters with enhanced charge transport properties†
Abstract
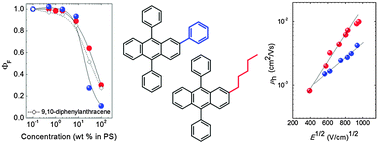
Realization of efficient deep-blue anthracene-based emitters with superior film-forming and charge transport properties is challenging. A series of non-symmetric 9,10-diphenylanthracenes (DPA) with phenyl and pentyl moieties at the 2nd position and alkyl groups at para positions of the 9,10-phenyls were synthesized and investigated. The non-symmetric substitution at the 2nd position enabled to improve film forming properties as compared to those of the unsubstituted DPA and resulted in glass transition temperatures of up to 92 °C. Small-sized and poorly conjugated substituents allowed to preserve emission in the deep blue range (<450 nm). Substitution at the 2nd position enabled to achieve high fluorescence quantum yields (up to 0.7 in solution, and up to 0.9 in the polymer host), although it caused an up to 10-fold increase in the intersystem crossing rate as compared to that of the unsubstituted DPA. Further optimization of the film forming properties achieved by varying the length of the alkyl groups attached at the 9,10-phenyls enabled to attain very high hole drift mobilities (∼5 × 10−3–1 × 10−2 cm2 V−1 s−1) in the solution-processed amorphous films of the DPA compounds.
 Please wait while we load your content...
Please wait while we load your content...