Spectroscopic characterization of C7H3+ and C7H3˙: electronic absorption and fluorescence in 6 K neon matrices†
Abstract
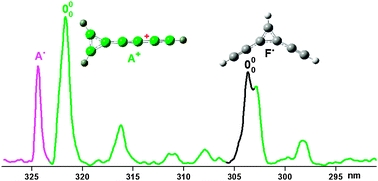
Mass selective deposition of C7H3+ (m/z = 87) into solid neon reveals the 11A1 ← X1A1 electronic absorption system of hepta-1,2,3,4,5,6-heptahexaenylium cation B+ [H2CCCCCCCH]+ with an origin band at 441.3 nm, 11A′ ← X1A′ transition of 2,4-pentadiynylium,1-ethynyl cation C+ [HCCCHCCCCH]+ starting at 414.6 nm and the 11A1 ← X1A1 one of cyclopropenylium,1,3-butadiynyl cation A+ [HCCCCC<(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)]+ with an onset at 322.2 nm. Vibrationally resolved fluorescence was observed for isomer B+ upon laser excitation of the absorption bands in the 11A1 ← X1A1 transition. After neutralization of the cations in the matrix five absorption systems of the C7H3 neutral radicals starting at 530.3, 479.4, 482.3, 325.0 and 302.5 nm were detected. These were identified as the 12A′ ← X2A′ and 22A′ ← X2A′ electronic transitions of 2-(buta-1,3-diynyl)cycloprop-2yl-1-1ylidene E˙ [HCCCCC<(C
CH)]+ with an onset at 322.2 nm. Vibrationally resolved fluorescence was observed for isomer B+ upon laser excitation of the absorption bands in the 11A1 ← X1A1 transition. After neutralization of the cations in the matrix five absorption systems of the C7H3 neutral radicals starting at 530.3, 479.4, 482.3, 325.0 and 302.5 nm were detected. These were identified as the 12A′ ← X2A′ and 22A′ ← X2A′ electronic transitions of 2-(buta-1,3-diynyl)cycloprop-2yl-1-1ylidene E˙ [HCCCCC<(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)]˙, 12B1 ← X2B1 of 1,2,3,4,5,6-heptahexaenyl B˙ [H2CCCCCCCH]˙, 32B1 ← X2B1 of 3-buta-1,3-diynyl-cyclopropenyl A˙ [HCCCCC<(CH
CH2)]˙, 12B1 ← X2B1 of 1,2,3,4,5,6-heptahexaenyl B˙ [H2CCCCCCCH]˙, 32B1 ← X2B1 of 3-buta-1,3-diynyl-cyclopropenyl A˙ [HCCCCC<(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)]˙ and 22B1 ← X2A2 transition of 1,2-divinylidene-cyclopropanyl radical F˙ [HCC-cyc-(CCHC)-CCH]˙, respectively. The assignment is based on calculated vertical excitation energies using the CASPT2 method. Comparison of the calculated harmonic vibrational frequencies with those inferred from the spectra supports the assignment.
CH)]˙ and 22B1 ← X2A2 transition of 1,2-divinylidene-cyclopropanyl radical F˙ [HCC-cyc-(CCHC)-CCH]˙, respectively. The assignment is based on calculated vertical excitation energies using the CASPT2 method. Comparison of the calculated harmonic vibrational frequencies with those inferred from the spectra supports the assignment.
 Please wait while we load your content...
Please wait while we load your content...