Site and bond-specific dynamics of reactions at the gas–liquid interface†
Abstract
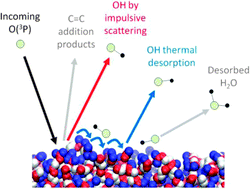
The dynamics of the interfacial reactions of O(3P) with the hydrocarbon liquids squalane (C30H62, 2,6,10,15,19,23-hexamethyltetracosane) and squalene (C30H50, trans-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene) have been studied experimentally. Laser-induced fluorescence (LIF) was used to detect the nascent gas-phase OH products. The O(3P) atoms are acutely sensitive to the chemical differences of the squalane and squalene surfaces. The larger exothermicity of abstraction from allylic C–H sites in squalene is reflected in markedly hotter OH rotational and vibrational distributions. There is a more modest increase in translational energy release. A larger fraction of the available energy is deposited in the liquid for squalene than for squalane, consistent with a more extensive geometry change on formation of the allylic radical co-product. Although the dominant reaction mechanism is direct, impulsive scattering, there is some evidence for OH being accommodated at both liquid surfaces, resulting in thermalised translation and rotational distributions. Despite the H-abstraction reaction being strongly favoured energetically for squalene, the yield of OH is substantially lower than for squalane. This is very likely due to competitive addition of O(3P) to the unsaturated sites in squalene, implying that double bonds are extensively exposed at the liquid surface.

 Please wait while we load your content...
Please wait while we load your content...