Halogen bond-assisted electron transfer reactions of aliphatic bromosubstituted electrophiles†
Abstract
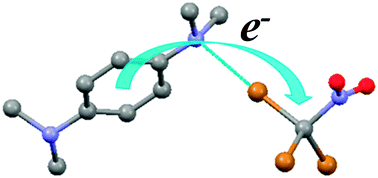
Isolation of pre-reactive complexes and analysis of kinetics of redox reactions of tetrabromomethane and tribromonitromethane demonstrated that halogen bonding results in significant lowering of the activation barriers for electron transfer, and that the rate constants of such (inner-sphere) processes can be evaluated from spectral, structural, and thermodynamic characteristics of the halogen-bonded associates.

 Please wait while we load your content...
Please wait while we load your content...