Non-equivalent conformations of d-amino acid oxidase dimer from porcine kidney between the two subunits. Molecular dynamics simulation and photoinduced electron transfer†
Abstract
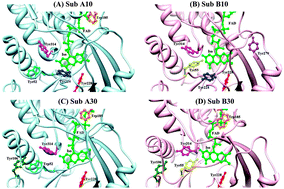
The structural difference between two subunits of D-amino acid oxidase dimer from porcine kidney was studied by molecular dynamics simulation (MDS) and rate of photoinduced electron transfer (ET) from aromatic amino acids as tyrosines (Tyr) and tryptophanes (Trp) to the excited isoalloxazine (Iso*). The donor–acceptor distances (Rc) between isoalloxazine (Iso) and the donors were shortest in Tyr224 (0.74 nm) in Sub A at 10 °C (Sub A10), in Tyr224 (0.79 nm) in Sub B at 10 °C (Sub B10), in Tyr228 (0.85 nm) in Sub A at 30 °C (Sub A30), and in Tyr224 (0.72 nm) in Sub B at 30 °C (Sub B30). The Rcs were mostly shorter in the dimer than those in the monomer. Hydrogen bonding (H-bond) pairs between Iso and surrounding amino acids varied with the subunit and temperature. O2 of the Iso ring formed an H-bond exclusively with Thr317OG1 (side chain) in both Sub A10 and Sub A30, while it formed with Gly315N (peptide), Leu316N and Thr317N in Sub B10 and Sub B30. N3H of Iso formed an H-bond with Leu51O (peptide) in Sub A10 and Sub A30, but not in Sub B10 and Sub B30. Electron affinity of Iso* was appreciably lower in Sub A10 compared to Sub B10, while it was opposite at 30 °C. ET rate to Iso* was fastest from Tyr224 in Sub A10, while it was fastest from Tyr314 in Sub B10. The ET rate was fastest from Tyr314 in Sub A30, while it was fastest from Tyr224 in Sub B30. The greater ET rates in the dimer as compared to those in the monomer were elucidated with shorter Rc in the dimer as compared to the monomer. The static dielectric constants inside the subunits and the static dielectric constant between Iso and Tyr224 or Tyr228 were not different appreciably. A few water molecules and sometimes an amino acid were located between Iso and Tyr224, which may be the reason why the dielectric constant of the entire subunits did not differ from that between Iso and Tyr224.

 Please wait while we load your content...
Please wait while we load your content...