Decoding the patterns of ubiquitin recognition by ubiquitin-associated domains from free energy simulations†
Abstract
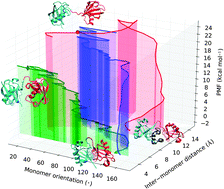
Ubiquitin is a highly conserved, highly represented protein acting as a regulating signal in numerous cellular processes. It leverages a single hydrophobic binding patch to recognize and bind a large variety of protein domains with remarkable specificity, but can also self-assemble into chains of poly-diubiquitin units in which these interfaces are sequestered, profoundly altering the individual monomers' recognition characteristics. Despite numerous studies, the origins of this varied specificity and the competition between substrates for the binding of the ubiquitin interface patch remain under heated debate. This study uses enhanced sampling all-atom molecular dynamics to simulate the unbinding of complexes of mono- or K48-linked diubiquitin bound to several ubiquitin-associated domains, providing insights into the mechanism and free energetics of ubiquitin recognition and binding. The implications for the subtle tradeoff between the stability of the polyubiquitin signal and its easy recognition by target protein assemblies are discussed, as is the enhanced affinity of the latter for long polyubiquitin chains compared to isolated mono- or diubiquitin.

 Please wait while we load your content...
Please wait while we load your content...