Polymorphism and isostructurality in sulfonylhydrazones†
Abstract
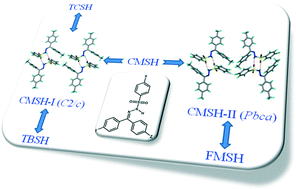
Five methyl and halogen derivatives of the conformationally flexible trimorphic molecule, bis(p-tolyl)ketone p-tosylhydrazone (TMSH, trimethyl sulfonylhydrazone) (Cryst. Growth Des., 2007, 7, 2047), were synthesized to understand polymorphism and isostructurality upon Cl–Me and inter-halogen exchange. The chlorodimethyl derivative CMSH (chlorodimethyl sulfonylhydrazone) is dimorphic whereas TCSH (trichloro sulfonylhydrazone), TBSH (tribromo sulfonylhydrazone), FMSH (fluorodimethyl sulfonylhydrazone), and MISH (methyldiiodo sulfonylhydrazone) have one crystal structure each. Single crystal X-ray diffraction and XPac analyses showed 3D isostructurality between CMSH form I, TCSH and TBSH, as well as for CMSH form II and FMSH. MISH has a different crystal packing compared to the other members due to the large iodo group. The conformational rigidity of the sulfonylhydrazone backbone leads to the observed isostructurality, whereas the presence of the sulfonamide dimer and catemer synthons results in different packing motifs and polymorphism.
- This article is part of the themed collection: International Year of Crystallography Celebration: India

 Please wait while we load your content...
Please wait while we load your content...