A ferrocene imidazolium-based macrocycle as an electrochemical chemosensor for halide anions†
Abstract
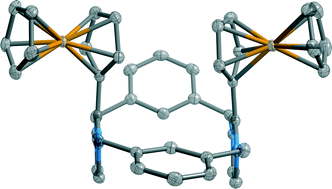
C4-H and C5-H protons of the imidazolium heterocycle have been exploited as a binding site for anion recognition in the design of two new redox-active imidazoliophane syn and anti isomers. The cooperative action of the two imidazolium motifs of the syn isomer is crucial for halide anion recognition. The integration of a ferrocene signalling unit at the C2 position of the imidazolium motif also facilitates the electrochemical sensing of anions by the syn isomer receptor.
- This article is part of the themed collection: Structural Macrocyclic Supramolecular Chemistry

 Please wait while we load your content...
Please wait while we load your content...