Large γ-CuI semiconductor single crystal growth by a temperature reduction method from an NH4I aqueous solution
Abstract
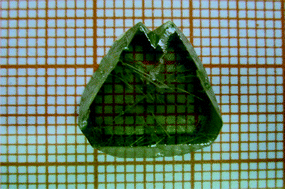
NH4I has been proven to be a promising cosolvent for cuprous iodide (CuI) single crystal growth from aqueous solutions by the temperature reduction method. In our work, as compared with NH4Cl and NH4Br, NH4I offers more advantages for single crystal growth, such as by remarkably increasing the solubility and growth rate of CuI crystals, effectively reducing the impurity concentration, and enhancing the crystal quality and crystallinity. A regular, centimeter-sized, high optical quality single crystal was successfully obtained using NH4I as a cosolvent. The electronic and optical properties of the as-grown crystal were characterized by Hall-effect measurements and optical transmission and photoluminescence spectra, respectively. The results demonstrated that the CuI crystal is conductive (high p-type mobility of 12.81 cm2 V−1 s−1) and transparent (great transmittance over 80%).

 Please wait while we load your content...
Please wait while we load your content...