Crystallisation control of paracetamol from ionic liquids
Abstract
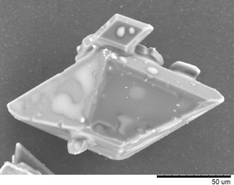
The crystallisation of Active Pharmaceutical Ingredients (API) from conventional organic solvents can yield undesirable crystal habits with poor physical properties that cause downstream processing problems. It has been proposed that ionic liquids (ILs) may offer an opportunity to perform controlled crystallisations from this media. Using paracetamol and the ionic liquids 1-butyl-3-methylimidazolium hexafluorophosphate [bmim][PF6] and 1-hexyl-3-methylimidazolium hexafluorophosphate [hmim][PF6] fundamental understanding of these systems was established by determining the Meta-stable Zone Width (MSZW) before completing a series of cooling crystallisations investigated across a temperature range between 20 °C and 90 °C. It has been shown that paracetamol can be crystallised from these systems to obtain the stable monoclinic form I and that the particle habit and size can be manipulated by changing the IL used, the solution concentration and the mechanism of crystal growth and in some cases crystal habits not commonly found from aqueous or organic solvents were produced. The results demonstrate that ILs may be a viable approach to manipulate crystal properties and should be explored more widely as a potential media for crystallisation of API.

 Please wait while we load your content...
Please wait while we load your content...