Synthesis, electron microscopy and X-ray characterization of oxymagnesite, MgO·2MgCO3, formed from amorphous magnesium carbonate
Abstract
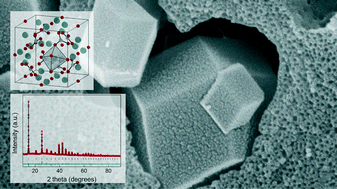
At present, the peculiar compound called oxymagnesite, MgO·2MgCO3, an intermediate formed during thermal decomposition of hydrated magnesium carbonates, has only been described a handful of times without a distinct description of its formation or morphology. In the current work we present the first scanning and transmission electron microscopy images of an oxymagnesite crystal together with its crystallographic data. Oxymagnesite was synthesized in a controlled manner via decomposition of amorphous magnesium carbonates (AMCs) subjected to varying relative humidity. We show that oxymagnesite is formed only when AMC is hydrated above a certain level, which we attribute to structural inequivalence between CO3 groups induced by water in AMC subjected to high humidity resulting in a weakening of some of the Mg–CO3 bonds. The study provides an understanding of the conditions needed for oxymagnesite formation and shows how hydrated AMCs can be used as precursors of different types of magnesium carbonates.

 Please wait while we load your content...
Please wait while we load your content...