Understanding the amino ↔ imino tautomeric preference in (imidazole)imidazolidine-N-aryl(alkyl) systems: a case study of moxonidine drug and insights from the Cambridge structural database (CSD)†
Abstract
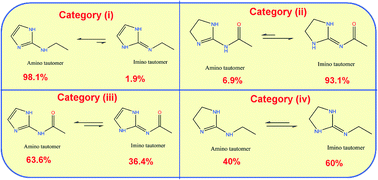
Crystals of moxonidine and its hydrochloride were grown in polar solvents and subjected to single crystal X-ray diffraction. The free base crystallizes in the monoclinic C2/c space group and the hydrochloride salt crystallizes as a monohydrate in the orthorhombic Pbca space group. Protonation in the HCl salt occurs at the imino N-atom of the 2-imino-imidazolidine group and results in clear changes in the bond angles and bond distances from the neutral structure. Both structures adopt perpendicular conformations and exhibit a certain degree of similarity in the way the homo and hetero dimer units are connected. Moxonidine can exhibit tautomerism along the imidazolidine-N-aryl fragment, but only the imino tautomer is observed in the crystal. Density functional theory (DFT) calculations in the gas phase suggest the amino tautomer to be highly unstable by 5.74 kcal mol−1 over the imino tautomer and this perhaps leads to its rare occurrence. 180 Crystal structures bearing a (imidazole)imidazolidine-N-aryl(alkyl) fragment were gathered from the Cambridge structural database (CSD) to understand if there are any molecular features, which can be correlated with the tautomeric occurrence in the crystal. We noticed that conjugation had a pronounced effect on the tautomer stability and its occurrence. Structures are divided into four categories based on the location of double bond towards the endo or exocyclic N atoms. In category (i), the amino tautomer is stabilized with a 98.1% success rate, because double bonds participate in conjugation towards the endocyclic N atoms. Conversely, in category (ii), conjugation is established towards the exocyclic N atoms and stabilizes the imino tautomer with 93.1% success. When conjugation is allowed towards the endo and exocyclic N sides (category (iii)) or is absent (category (iv)), both amino and imino tautomers are observed. Other important factors such as intramolecular and intermolecular hydrogen bonding in the crystal have shown to influence the tautomeric existence and we illustrate these features through selected examples. Tautomer energy calculations were performed on selected examples from the four categories and their relative stabilities correlated with their observation in the crystals. We also demonstrate how the in-depth structural analysis of the CSD helps us to revise two incorrectly determined structures reported earlier. The amino tautomeric form of XIBPOO is corrected to its protonated form with its co-former formamide corrected as formate anion in its crystal lattice. Similarly, the amino tautomeric form of QABDON is corrected to its imino tautomer.

 Please wait while we load your content...
Please wait while we load your content...