Structures of NHC Hg(ii) and Ag(i) complexes and selective recognition of nitrate anion†
Abstract
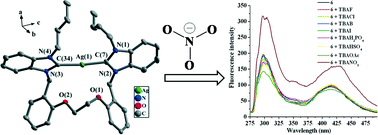
A series of bis-benzimidazolium (or bis-imidazolium) salts, and their eight N-heterocyclic carbene mercury(II) and silver(I) complexes (1–8) have been synthesized and characterized. Complexes 1–4 and 6 contain similar macrometallocycles formed via one bidentate carbene ligand and one metal ion. Their major differences are their anionic units, which are related to the amount of metal sources added. In complex 7, one 30-membered macrometallocycle is generated via two ligands and two silver(I) atoms in dilute solution. For the open structure of complexes 5 and 8, 5 contains an anionic unit [Hg3Cl8]2−, and 8 is formed as a dimer through two [L3Ag2Cl2] monomers. The study of selective recognition of anions on the basis of fluorescence and UV/vis spectroscopic titrations indicates that macrometallocycle 6 is an effective chemosensor for nitrate anion.

 Please wait while we load your content...
Please wait while we load your content...