Polymorphism of felodipine co-crystals with 4,4′-bipyridine†
Abstract
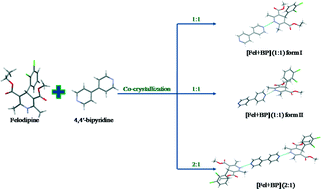
The calcium-channel blocking agent felodipine (Fel) forms co-crystals with 4,4′-bipyridine (BP) with 1 : 1 and 2 : 1 molar ratios. The [Fel + BP] (1 : 1) co-crystal exists in two polymorphic forms. Differential scanning calorimetry and solution calorimetry show that form I of the [Fel + BP] (1 : 1) co-crystal is the most thermodynamically stable phase. The difference in the crystal lattice energies between different polymorphs of the co-crystal is found to be comparable with that between the polymorphic forms of pure felodipine. The enthalpies of formation of the co-crystals are small, which indicates that the packing energy gain originates from only weak van der Waals interactions. Analysis of Hirshfeld surfaces of the felodipine molecule shows a similar distribution of intermolecular contacts in the co-crystals and pure felodipine.

 Please wait while we load your content...
Please wait while we load your content...