Electroactive tetrathiafulvalene based pyridine-mono and -bis(1,2,3-triazoles) click ligands: synthesis, crystal structures and coordination chemistry†‡
Abstract
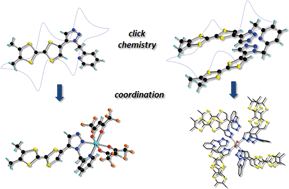
Electroactive ligands picoline-1,2,3-triazole-5-DM-TTF and lutidine-bis(1,2,3-triazole-5-DM-TTF) are synthesized by click chemistry following the ruthenium catalyzed azide–alkyne cycloaddition strategy. In the solid state structure of the bis(triazole) derivative, a tweezer conformation is observed, with the two TTF units arranged in a parallel manner. Cyclic voltammetry measurements show a splitting of the first oxidation wave, demonstrating sequential oxidation of the TTF donors in the intramolecular in through space mixed valence radical cation and then dication, suggesting that the tweezer conformation is maintained also in solution. A cobalt(II) complex based on the chelating picoline-triazole ligand is described. The lutidine-bis-triazole ligand shows no chelating behavior in the two structurally reported cadmium(II) complexes. In one of the complexes, the Cd(II) center is coordinated by four triazole rings from four different ligands, while the other four triazole units establish hydrogen bonds with one axially coordinated water molecule. The second complex is a one-dimensional coordination polymer, containing the CdCl2 fragment, in which the repeating motif is a pentanuclear “Cd5Cl10” unit decorated with two TTF ligands coordinated in a ditopical mode through the triazole units. The TTF donors establish intra- and intermolecular S⋯S contacts.

 Please wait while we load your content...
Please wait while we load your content...