C–H/O interactions of nucleic bases with a water molecule: a crystallographic and quantum chemical study†
Abstract
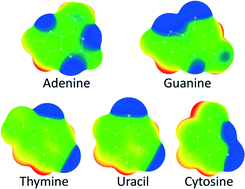
The C–H/O interactions of nucleic bases with a water molecule were studied by analyzing data in the Cambridge Structural Database (CSD) and by ab initio calculations. The analysis of the C–H/O interactions in the crystal structures from the CSD indicates that nucleic base–water C–H/O interactions do not show preference for linear contacts. The results of the ab initio calculations are in accord with the CSD data and show that the bifurcated C–H/N–H interactions are stronger than linear interactions for all nucleic bases. The bifurcated C–H/N–H interactions are also stronger than the bifurcated C–H/C–H interactions. The strongest interaction is the bifurcated C6–H/N1–H interaction of uracil with an energy of −5.46 kcal mol−1 calculated at the MP2/cc-pVTZ level. All linear C–H/O interactions, except one with adenine, are stronger than −2.0 kcal mol−1. The strongest linear interaction is with uracil, −3.59 kcal mol−1. The calculated electrostatic potential maps for nucleic base molecules can explain the results we obtained for interaction energies. The results show that C–H/O interactions of nucleic bases with a water molecule are substantially stronger than C–H/O interactions of benzene (−1.28 kcal mol−1) and pyridine (−1.97 kcal mol−1). The investigation of C–H/O interactions of nucleic bases with water could shed light on the intermolecular interactions of DNA or RNA bases with other molecules.

 Please wait while we load your content...
Please wait while we load your content...