Transition metal ion-assisted synthesis of monodisperse, quasi-spherical gold nanocrystals via citrate reduction†
Abstract
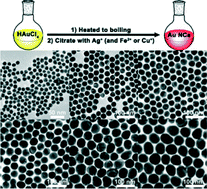
We demonstrate that monodisperse, quasi-spherical gold nanocrystals (Au NCs) can be produced via quick addition of an aqueous solution of sodium citrate containing Ag+ and Fe2+ or Cu+ ions into a boiling aqueous solution of HAuCl4. The size of the resulting Au NCs increases from 12 nm to 60 nm with the decrease of citrate concentration. Compared with those obtained via a conventional Frens method, the NCs obtained via our approach have a fairly narrow distributed size and spherical shape. The significant improvement in the size polydispersity and shape sphericity is a result of the synergic effect of Ag+ and Fe2+ (or Cu+) ions. During the formation of Au NCs, the Ag+ ions are utilized to accelerate the nucleation via catalysis of the oxidation of citrate and regulate the NC isotropic growth, while the Fe2+ (or Cu+) ions significantly accelerate the reduction of Au3+ ions to Au0 atoms to speed up the nucleation when the concentration of citrate and silver ions was reduced to below 6.0 × 10−3 wt% and 8.5 × 10−4 wt%, respectively.

 Please wait while we load your content...
Please wait while we load your content...