Synthesis, structure, topology and magnetic properties of new coordination polymers based on 5(–Br/–COOH)-substituted nicotinic acid†
Abstract
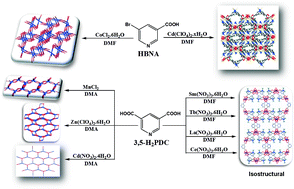
Nine new coordination polymers of 5-substituted nicotinic acid (with either –Br, HBNA, or –COOH, 3,5-H2PDC) with d-transition (Co, Ni, Zn and Cd) and f-lanthanoid (La, Ce, Sm and Tb) metal ions have been synthesized. The two metal complexes [Co2(BNA)4(H2O)]n·H2O (1a) and [Cd(BNA)2]n (1b) have polymorphic/isomeric structures to those of metal–organic-frameworks (MOFs) with similar structural building units (SBUs) reported earlier. Modified reaction conditions yielded new isomeric metal complexes in the present case. Reactions of the 3,5-H2PDC linker with different metal ions led to the formation of polymeric complexes [Mn3(3,5-PDC)3(DMA)3]n·DMA (2a), [Zn(3,5-PDC)(DMA)]n·DMA (2b) and [Cd2(3,5-PDC)2(DMA)4]n (2c), as well as lanthanoid MOFs [Sm(3,5-PDC)1.5(DMF)]n·DMF (2d, as well as isomorphous products with La (2f) and Ce (2g) ions) and [Tb2(3,5-PDC)3(DMF)2]n·DMF (2e). Networking diversity observed in these coordination polymers with the conformationally rigid 5-substituted nicotinic acid ligand is discussed. The magnetic properties of 2a, 2d and 2e have been studied as well.

 Please wait while we load your content...
Please wait while we load your content...