Interplay between hydrogen bonding and metal coordination in alkali metal tartrates and hydrogen tartrates†
Abstract
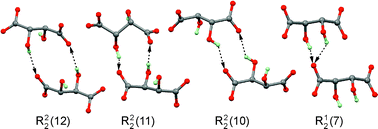
The reactions of D,L-tartaric acid (D,L-H2Tart) with alkali metal hydroxides MOH (M = Li, Na, Rb, Cs) in aqueous solution yielded new polymorphs of Na(D,L-HTart)·H2O (1a) and Cs(D,L-HTart) (2a) as well as crystals of LiCs(D,L-Tart)·2H2O (3) and the conglomerate (Rb0.5Cs0.5)2(D-Tart) (4)/(Rb0.5Cs0.5)2(L-Tart). The crystal structures of these salts display 1D (1a, 2a), 2D (3) or 3D (4) coordination polymers in combination with a hydrogen bonded layer (1a), framework (2a, 4) or tape (3) structure. The triclinic and monoclinic polymorphs of Na(D,L-HTart)·H2O (1) exhibit a close one-dimensional packing similarity. Standard O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bond motifs for the aggregation of HTart− and Tart2− ions in the solid state were identified from a comparison involving 35 crystal structures of chiral and racemic MHTart and M2Tart salts (M = alkali metal).
C hydrogen bond motifs for the aggregation of HTart− and Tart2− ions in the solid state were identified from a comparison involving 35 crystal structures of chiral and racemic MHTart and M2Tart salts (M = alkali metal).

 Please wait while we load your content...
Please wait while we load your content...