Three metal–organic frameworks based on the semirigid V-shaped 5-(3-amino-tetrazole-5-phenoxy)-isophthalic acid ligand: syntheses, topological structures and properties†
Abstract
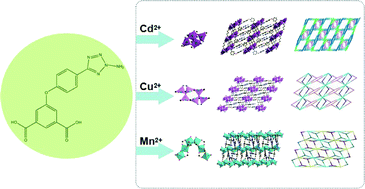
The designed organic ligand 5-(3-amino-tetrazole-5-phenoxy)-isophthalic acid (H2atpia) has been successfully prepared and its coordination features have been explored. By the reaction of H2atpia with transition metals, three metal–organic frameworks (MOFs), namely [Cd(atpia)]2 (1), [Cu3O2(atpia)2] (2) and [Mn3(OH)2(atpia)3]·1.25H2O (3) have been synthesized under hydrothermal conditions. All the compounds were fully characterized by elemental analysis, IR spectroscopy, thermogravimetric analysis, power X-ray diffraction and single-crystal X-ray diffraction. These three compounds all display new topologies. Compound 1 exhibits a 3D (2,3,10)-connected framework with (4·62)2(412·618·811·104)(4)2 topology. Compound 2 possesses a 2D 3-nodal layer with (416·614·811·104)(43)2(4)2 topology. Compound 3 features a 2D (3,3,8,8)-connected structure with unprecedented (3·42)2(34·46·56·68·73·8) topology. The diverse structures of these three compounds demonstrate that the distinctive coordination modes of different metals have a significant impact on the construction of MOFs. Moreover, the photoluminescence properties of 1 and the magnetic properties of 2 and 3 have been studied and discussed.

 Please wait while we load your content...
Please wait while we load your content...