Three 3D lanthanide–organic frameworks with sra topology: syntheses, structures, luminescence and magnetic properties†
Abstract
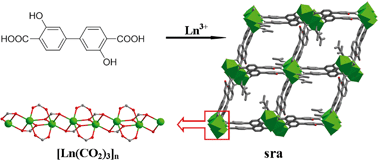
Three 3D lanthanide–organic frameworks (LOFs), [Ln(HL)(DMF)]·2DMF (Ln = Eu (1), Gd (2), Dy (3); H4L = 3,3′-dihydroxy-4,4′-biphenyldicarboxylic acid), have been prepared by solvothermal reaction of Ln(NO3)3·6H2O and H4L in DMF–H2O mixed solvent. Crystallographic data show that 1–3 are isomorphous and crystallize in the monoclinic space group P21/c. Each Ln(III) is eight-coordinated to seven O atoms from five (HL)3− ligands and one O atom from a DMF molecule in a dodecahedron geometry. The adjacent Ln(III) ions are linked by the carboxylate groups of the (HL)3− ligands to form a 1D inorganic rod-shaped [Ln(CO2)3]n chain as a secondary building unit (SBU). The infinite 1D chains are interconnected by the biphenyl groups, giving rise to a 3D sra framework containing 1D open rhombic channels with dimensions of 18.4 × 11.6 Å along the a axis. The present LOF is the first sra-net built from Ln–O–C rods with only one ligand. The ligand (HL)3− shows an unusual septadentate coordination mode with one bound hydroxy group. Furthermore, 1 exhibits characteristic luminescence of Eu3+ upon 394 nm excitation. The investigation of magnetic properties reveals the weak antiferromagnetic interactions (J1 = −0.018(14) and J2 = −0.021(13) cm−1) between Gd(III) ions in 2 and θ = −1.62(2) K in 3 due to thermal depopulation of the Stark levels of Dy(III) ions and/or the possible antiferromagnetic interactions between Dy(III) ions.

 Please wait while we load your content...
Please wait while we load your content...