Synthesis of graphene oxide–Ag2CO3 composites with improved photoactivity and anti-photocorrosion†
Abstract
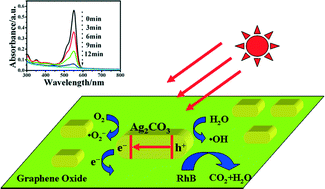
Graphene oxide–Ag2CO3 (GO–Ag2CO3) composites have been prepared by a simple and effective precipitation method, and found to exhibit a higher photocatalytic activity toward degradation of organic dyes, including rhodamine B, methylene blue and methyl orange, in aqueous solution, than pure Ag2CO3 crystal under visible light. The Ag2CO3 crystal with 0.9 wt% GO content exhibited the highest photodegradation efficiency for organic dyes, which was 2 times that of pure Ag2CO3 crystal. The improved photocatalytic activity of GO–Ag2CO3 composites was attributed to the corporative effects of high-surface-area of GO sheets, enhanced absorption of organic dyes, small band gap, more efficient separation of photogenerated electron–hole pairs, and good electron acceptor properties of GO. The photocorrosion of Ag2CO3 crystal can be efficiently inhibited by GO, since the transfer of photogenerated electrons from the surface of Ag2CO3 crystal to GO sheets can reduce the possibility of decomposing Ag+ to Ag0, which resulted in an improved stability and recyclability of the GO–Ag2CO3 composite in the photocatalytic reaction. This facile and straightforward method has promising applications in the fabrication of different graphene oxide-based heterostructure photocatalysts.

 Please wait while we load your content...
Please wait while we load your content...