Protein secondary-shell interactions enhance the photoinduced hydrogen production of cobalt protoporphyrin IX†
Abstract
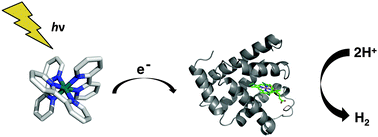
Hydrogen is an attractive fuel with potential for production scalability, provided that inexpensive, efficient molecular catalysts utilizing base metals can be developed for hydrogen production. Here we show for the first time that cobalt myoglobin (CoMyo) catalyzes hydrogen production in mild aerobic conditions with turnover number of 520 over 8 hours. Compared to free Co-protoporphyrin IX, incorporation into the myoglobin scaffold results in a 4-fold increase in photoinduced hydrogen production activity. Engineered variants in which specific histidine resides in proximity of the active site were mutated to alanine result in modulation of the catalytic activity, with the H64A/H97A mutant displaying activity 2.5-fold higher than wild type. Our results demonstrate that protein scaffolds can augment and modulate the intrinsic catalytic activity of molecular hydrogen production catalysts.

 Please wait while we load your content...
Please wait while we load your content...