Zinc-mediated CH-activation of tetrahydrofuran under mild conditions for the regioselective addition to aryl-propiolates†
Abstract
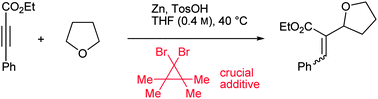
The CH-activation of THF is realized in a zinc-mediated process using a dibromocyclopropane as a crucial additive. The highly regioselective addition to aryl-substituted propiolates as well as the regio- and stereoselective addition to diynes are described.

 Please wait while we load your content...
Please wait while we load your content...