Iron-catalysed asymmetric tandem spiro-cyclization using dioxygen in air as the hydrogen acceptor†
Abstract
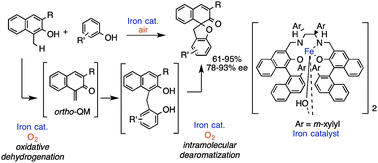
A tandem combination of ortho-quinone methide (o-QM) formation/Michael addition/asymmetric dearomatization, which is catalysed by an iron–salan complex in air with high enantioselectivity, provides an efficient method for spirocyclic (2H)-dihydrobenzofuran synthesis from 2-naphthols and phenols. The key to the success of the tandem synthesis is the development of aerobic oxidative o-QM formation.

 Please wait while we load your content...
Please wait while we load your content...