Temperature controlled invertible selectivity for adsorption of N2 and CH4 by molecular trapdoor chabazites†
Abstract
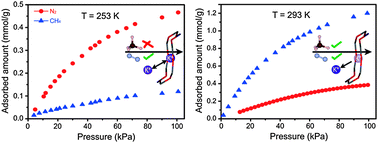
We report an unusual operating regime for a chabazite zeolite in which the adsorption selectivity for N2 over CH4 inverts from being more selective for N2 at 253 K, to becoming less selective with increasing temperature and eventually becoming selective for CH4 over N2 above 293 K.

 Please wait while we load your content...
Please wait while we load your content...