Dioxygen activation by an organometallic Pd(ii) precursor: formation of a Pd(iv)–OH complex and its C–O bond formation reactivity†
Abstract
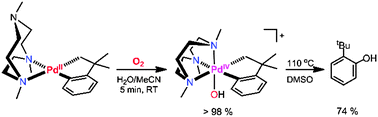
The complex (Me3tacn)PdII(CH2CMe2C6H4) is readily oxidized by O2 or H2O2 to yield the PdIV–OH complex [(Me3tacn)PdIV(OH)(CH2CMe2C6H4)]+. Thermolysis of this product leads to the selective C(sp2)–O reductive elimination of 2-tert-butylphenol, no C(sp3)–O elimination product being detected. This system represents a rare example of selective C(sp2)–O bond formation that is relevant to Pd-catalyzed aerobic C–H hydroxylation reactions.
- This article is part of the themed collection: Metal-Mediated Transformations of Small Molecules

 Please wait while we load your content...
Please wait while we load your content...