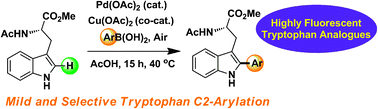
A mild and selective Pd-mediated methodology for the synthesis of highly fluorescent 2-arylated tryptophans and tryptophan-containing peptides: a catalytic role for Pd0 nanoparticles?†
Abstract
A Pd-mediated direct C–H bond functionalisation of tryptophan has been developed, both as a single amino acid residue and within peptides. Important mechanistic insight into this process has been gained by characterising a Pd catalytically competent nanoparticle phase which evolves during the early stages of reaction.

 Please wait while we load your content...
Please wait while we load your content...