Production of hydrogen by electrocatalysis: making the H–H bond by combining protons and hydrides
Abstract
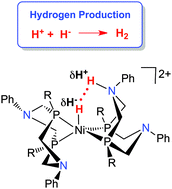
Generation of hydrogen by reduction of two protons by two electrons can be catalysed by molecular electrocatalysts. Determination of the thermodynamic driving force for elimination of H2 from molecular complexes is important for the rational design of molecular electrocatalysts, and allows the design of metal complexes of abundant, inexpensive metals rather than precious metals (“Cheap Metals for Noble Tasks”). The rate of H2 evolution can be dramatically accelerated by incorporating pendant amines into diphosphine ligands. These pendant amines in the second coordination sphere function as protons relays, accelerating intramolecular and intermolecular proton transfer reactions. The thermodynamics of hydride transfer from metal hydrides and the acidity of protonated pendant amines (pKa of N–H) contribute to the thermodynamics of elimination of H2; both of the hydricity and acidity can be systematically varied by changing the substituents on the ligands. A series of Ni(II) electrocatalysts with pendant amines have been developed. In addition to the thermochemical considerations, the catalytic rate is strongly influenced by the ability to deliver protons to the correct location of the pendant amine. Protonation of the amine endo to the metal leads to the N–H being positioned appropriately to favor rapid heterocoupling with the M–H. Designing ligands that include proton relays that are properly positioned and thermodynamically tuned is a key principle for molecular electrocatalysts for H2 production as well as for other multi-proton, multi-electron reactions important for energy conversions.
- This article is part of the themed collection: Electrocatalytic hydrogen evolution

 Please wait while we load your content...
Please wait while we load your content...