Metal-free Lewis acid mediated dehydrocoupling of phosphines and concurrent hydrogenation†
Abstract
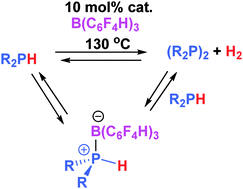
The stoichiometric reaction of trityl cation with two equivalents of Ph2PH affords the phosphine stabilized phosphenium salt [Ph2(H)PPPh2][B(C6F5)4] via hydride abstraction, while catalytic amounts of B(p-HC6F4)3 effects catalytic phosphine dehydrocoupling with the liberation of H2. This reaction is accelerated by the presence of olefin or imine, effecting concurrent hydrogenation.

 Please wait while we load your content...
Please wait while we load your content...