Sulfide synthesis through copper-catalyzed C–S bond formation under biomolecule-compatible conditions†
Abstract
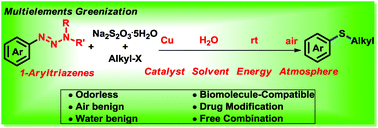
We report here an efficient and mild method for constructing C–S bonds. The reactions were carried out with Na2S2O3 as a sulfurating reagent, CuSO4 as a catalyst, and water as solvent without any surfactant. The products were achieved in moderate to excellent yields at room temperature under air. Notably, this reaction is compatible with various biomolecules including amino acids, oligosaccharides, nucleosides, proteins, and cell lysates.

 Please wait while we load your content...
Please wait while we load your content...