2-Arylcyclopropylmethanol as a substitute for homoallyl aryl alcohol in the construction of cis-2,6-disubstituted tetrahydropyran: synthesis of (±)-centrolobine†
Abstract
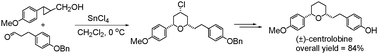
The application of 2-arylcyclopropylmethanols as substitutes to homoallyl aryl alcohols and their reactions with aliphatic aldehydes in the presence of SnCl4 in CH2Cl2 leads to an efficient Prins cyclization to generate cis-2,6-disubstituted tetrahydropyrans in high yields. The reaction is free from 2-oxonia-Cope rearrangement. This protocol was used to synthesize (±)-centrolobine in an overall 84% yield over three steps. The protocol holds promise for scaffold generation for medicinal chemistry exploitation.

 Please wait while we load your content...
Please wait while we load your content...