NBS-promoted oxidation of fullerene monoradicals leading to regioselective 1,4-difunctional fullerenes†
Abstract
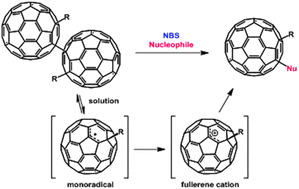
We have demonstrated that NBS is able to promote the oxidation of fullerene monoradicals to form 1,4-difunctional fullerenes. The singly bonded fullerene dimers were used as fullerene monoradical precursors, which produced various 1,4-fullerenes with a wide range of functional groups in good to high yields with high regioselectivity in terms of cosolvents and nucleophiles.


 Please wait while we load your content...
Please wait while we load your content...