A highly efficient asymmetric synthesis of quaternary stereocenter-containing indolizidine and quinolizidine alkaloids using aldehydes, nitroalkenes, and unactivated cyclic ketimines†
Abstract
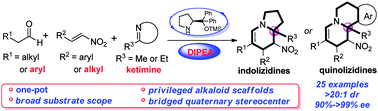
A highly efficient approach for the construction of indolizidines and quinolizidines bearing a bridged quaternary stereocenter has been established in a one-pot fashion using aldehydes, nitroalkenes, and cyclic ketimines with excellent enantioselectivities and in high yields. Moreover, this method could be applied to the synthesis of indolizidines in the gram scale.

 Please wait while we load your content...
Please wait while we load your content...