Multicomponent decarboxylative allylations†
Abstract
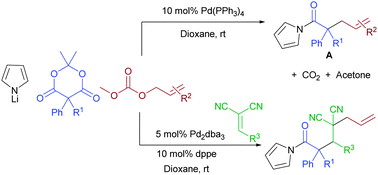
The reaction of Meldrum's acid, pyrrolide, and allyl carbonates allows a multicomponent decarboxylative coupling to form allylated acyl pyrroles. This strategy is made possible by the in situ formation of β-oxo carboxylates from Meldrum's acid. Subsequent decarboxylative enolate formation and electrophilic allylation complete the reaction. Addition of benzylidene malononitriles as good Michael acceptors allow a 4-component interceptive decarboxylative allylation.

 Please wait while we load your content...
Please wait while we load your content...