Cyclization of alkynoic acids in water in the presence of a vesicular self-assembled amphiphilic pincer palladium complex catalyst†
Abstract
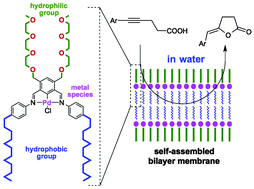
Alkynoic acids were cyclized in the presence of a vesicular palladium-based catalyst and a catalytic amount of triethylamine in water to give the corresponding lactones in moderate-to-good yields. The formation of a vesicular structure was shown to be essential for the promotion of the reaction.

 Please wait while we load your content...
Please wait while we load your content...