A cation-directed two-component cascade approach to enantioenriched pyrroloindolines†
Abstract
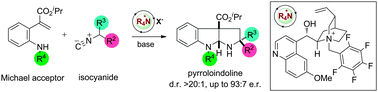
A cascade approach to complex pyrroloindolines bearing all-carbon quaternary stereocentres has been developed. This two-component process uses a chiral ammonium salt to control diastereo- and enantioselectivity in the addition of isocyanides to functionalized alkenes to afford pyrroloindolines with up to three stereocentres. A mechanistic proposal involving intramolecular hydrogen bond activation of the isocyanide is described.

 Please wait while we load your content...
Please wait while we load your content...