QM/MM simulations as an assay for carbapenemase activity in class A β-lactamases†
Abstract
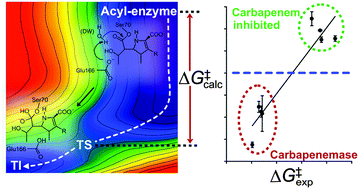
Carbapenems, ‘last resort’ antibiotics for many bacterial infections, can now be broken down by several class A β-lactamases (i.e. carbapenemases). Here, carbapenemase activity is predicted through QM/MM dynamics simulations of acyl–enzyme deacylation, requiring only the 3D structure of the apo-enzyme. This may assist in anticipating resistance and future antibiotic design.


 Please wait while we load your content...
Please wait while we load your content...