Double oxidation of α-(alkylideneamino)nitriles to imides by molecular oxygen under mild basic conditions†
Abstract
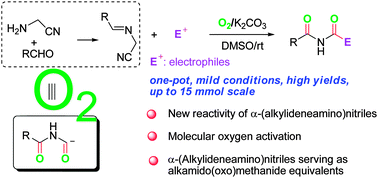
We reveal here the unique reactivity of α-(alkylideneamino)nitriles toward molecular oxygen. Thus, α-(alkylideneamino)nitriles can serve as the imide building block for the efficient synthesis of imides in the absence of transition metals under extremely mild conditions.

 Please wait while we load your content...
Please wait while we load your content...