Thermodynamics of halogen bonded monolayer self-assembly at the liquid–solid interface†
Abstract
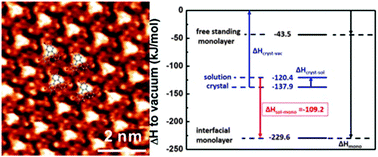
Monolayer self-assembly of a hexabrominated, three-fold symmetric aromatic molecule is studied at the heptanoic acid–graphite interface. Thermodynamical insights are obtained from an adapted Born–Haber cycle that is utilized to derive the overall enthalpy change including solvent effects. Comparison with theoretical entropy estimates suggests a minor influence of solvation.

 Please wait while we load your content...
Please wait while we load your content...