Stereoselective synthesis of γ-hydroxynorvaline through combination of organo- and biocatalysis†
Abstract
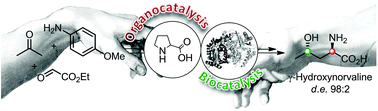
An efficient route for the synthesis of all four diastereomers of PMP-protected α-amino-γ-butyrolacton to access γ-hydroxynorvaline was established. The asymmetric key steps comprise an organocatalytic Mannich reaction and an enzymatic ketone reduction. Three reaction steps could be integrated in a one-pot process, using 2-PrOH both as solvent and as reducing agent. The sequential construction of stereogenic centres gave access to each of the four stereoisomers in high yield and with excellent stereocontrol.

 Please wait while we load your content...
Please wait while we load your content...